CYBERLYNK was created in response to a persistent and costly problem observed across pharmaceutical manufacturing projects: critical integration issues are routinely identified far too late in the project lifecycle.
After years of hands-on experience delivering commissioning, automation, and IT systems within regulated manufacturing environments, it became clear that Factory Acceptance Testing (FAT) and Site Acceptance Testing (SAT) were fundamentally misaligned with how modern, software-driven production lines actually behave. Integration between machines and higher-level systems was often only validated once equipment arrived on site — when timelines are compressed, costs escalate, and risk is highest.
CYBERLYNK was developed to close this gap by enabling secure, real-world system integration testing before equipment delivery.
In traditional projects:
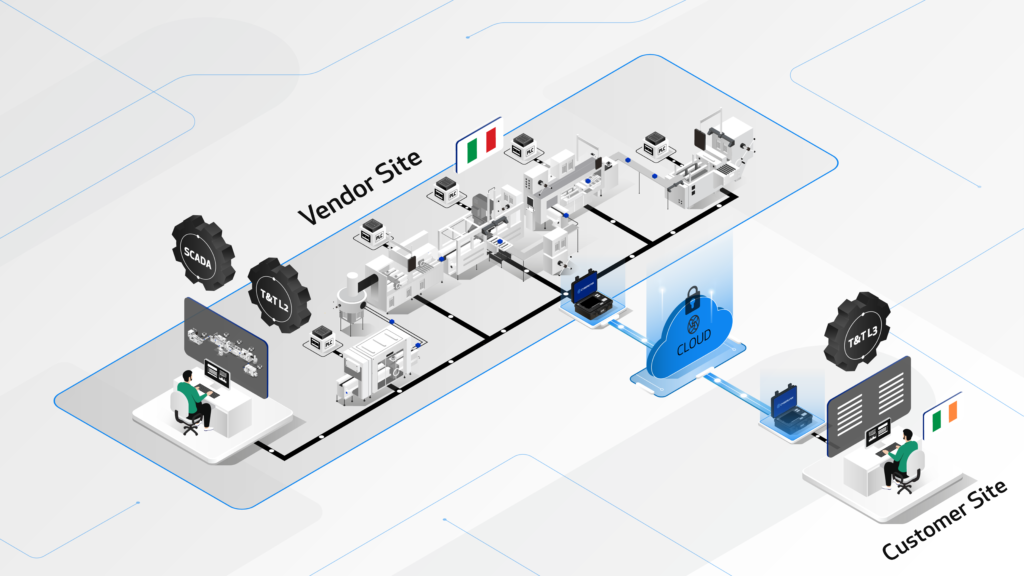
Factory Acceptance Testing (FAT) verifies that individual machines function as specified at the vendor site.
Site Acceptance Testing (SAT) tests that machines and software systems work together once installed at the customer site.
However, as production environments have become increasingly software-driven — with MES, SCADA, Track & Trace, and enterprise systems tightly coupled — many of the most critical system integration issues only surface during SAT. At that stage, resolving issues often requires on-site troubleshooting, specialist travel, rework, and unplanned installation downtime.
This late-stage discovery introduces unnecessary risk, delays time-to-production, and reduces confidence in project delivery.
CYBERLYNK fundamentally changes when and where integration risk is addressed.
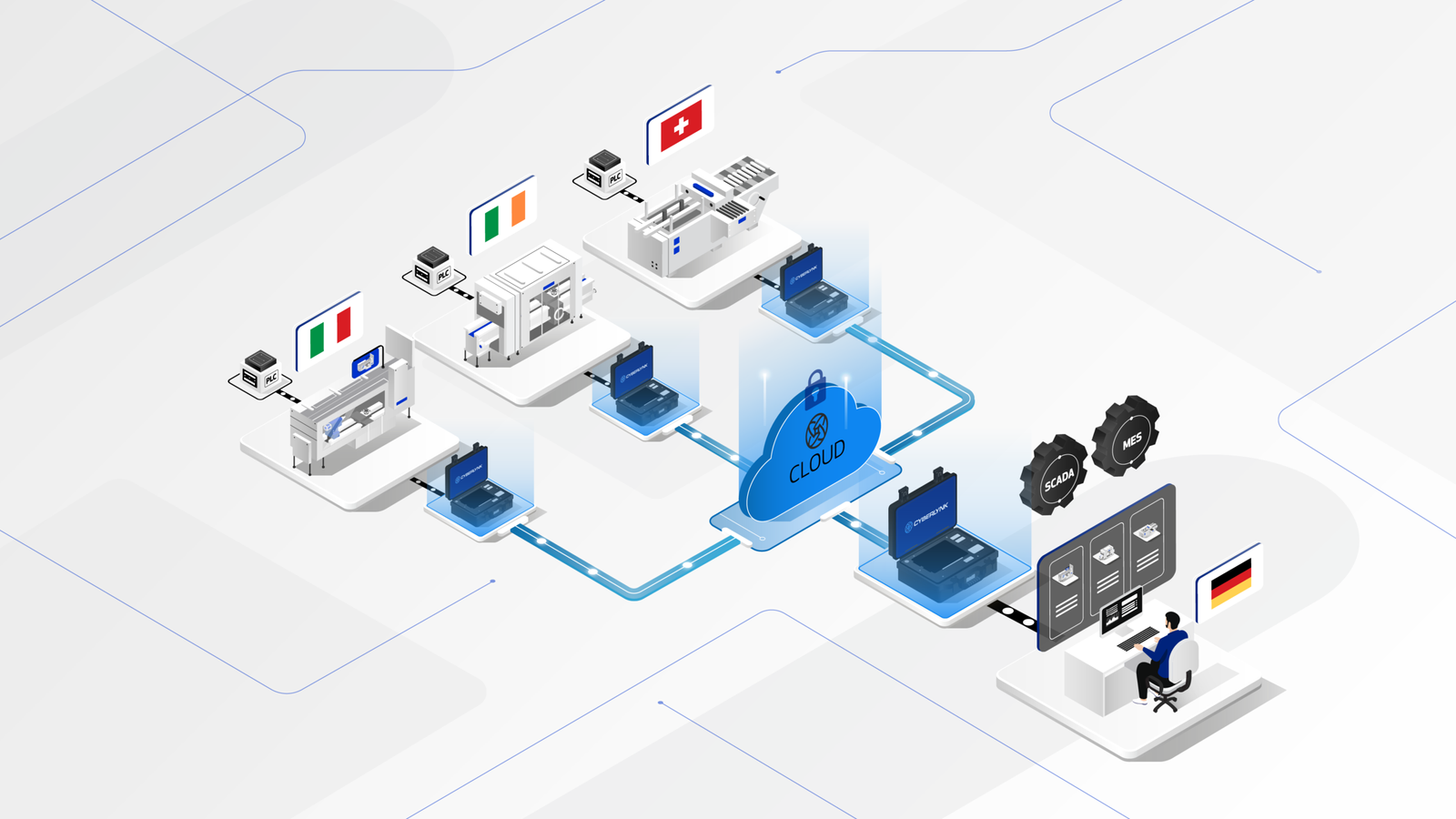
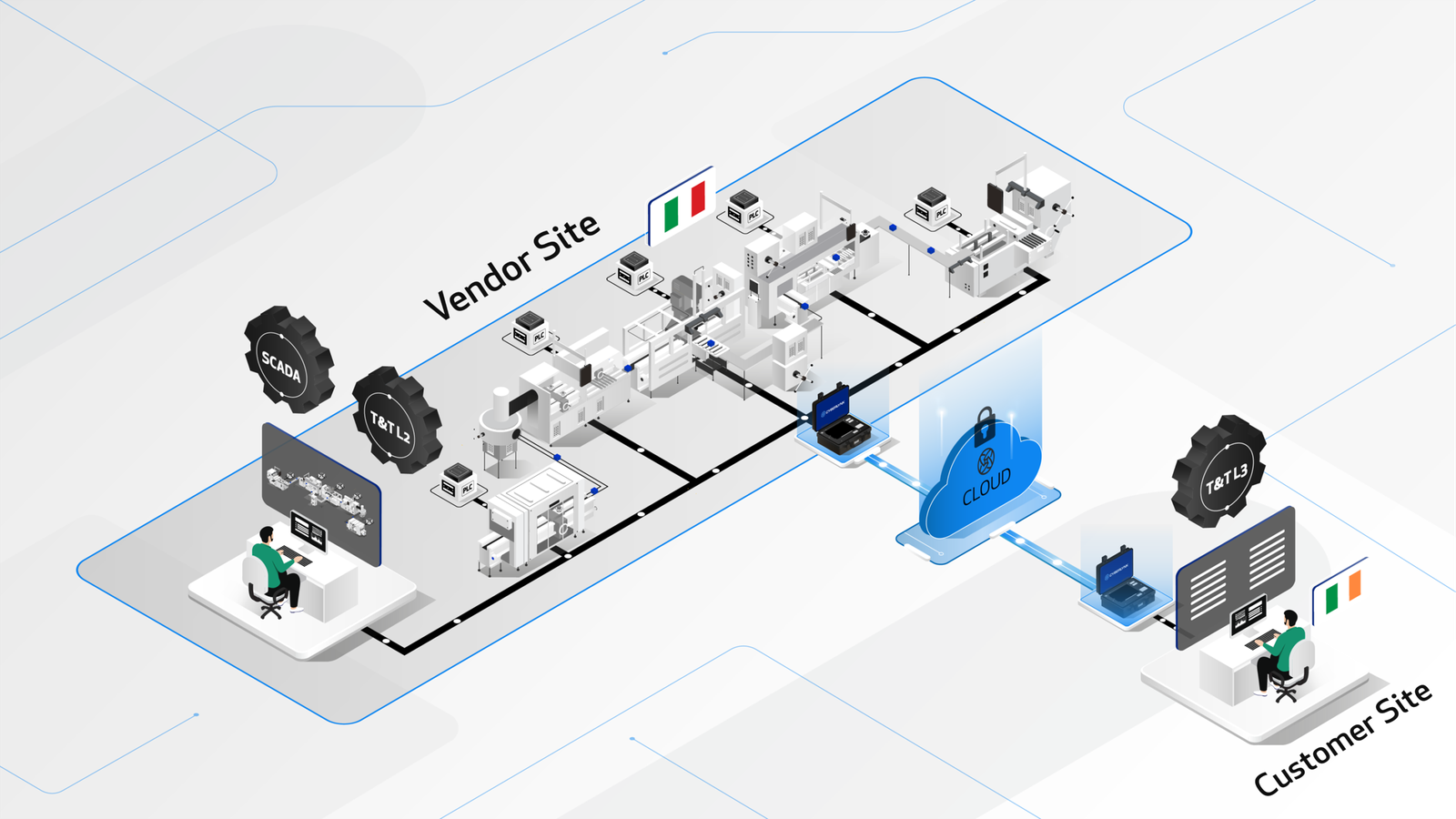
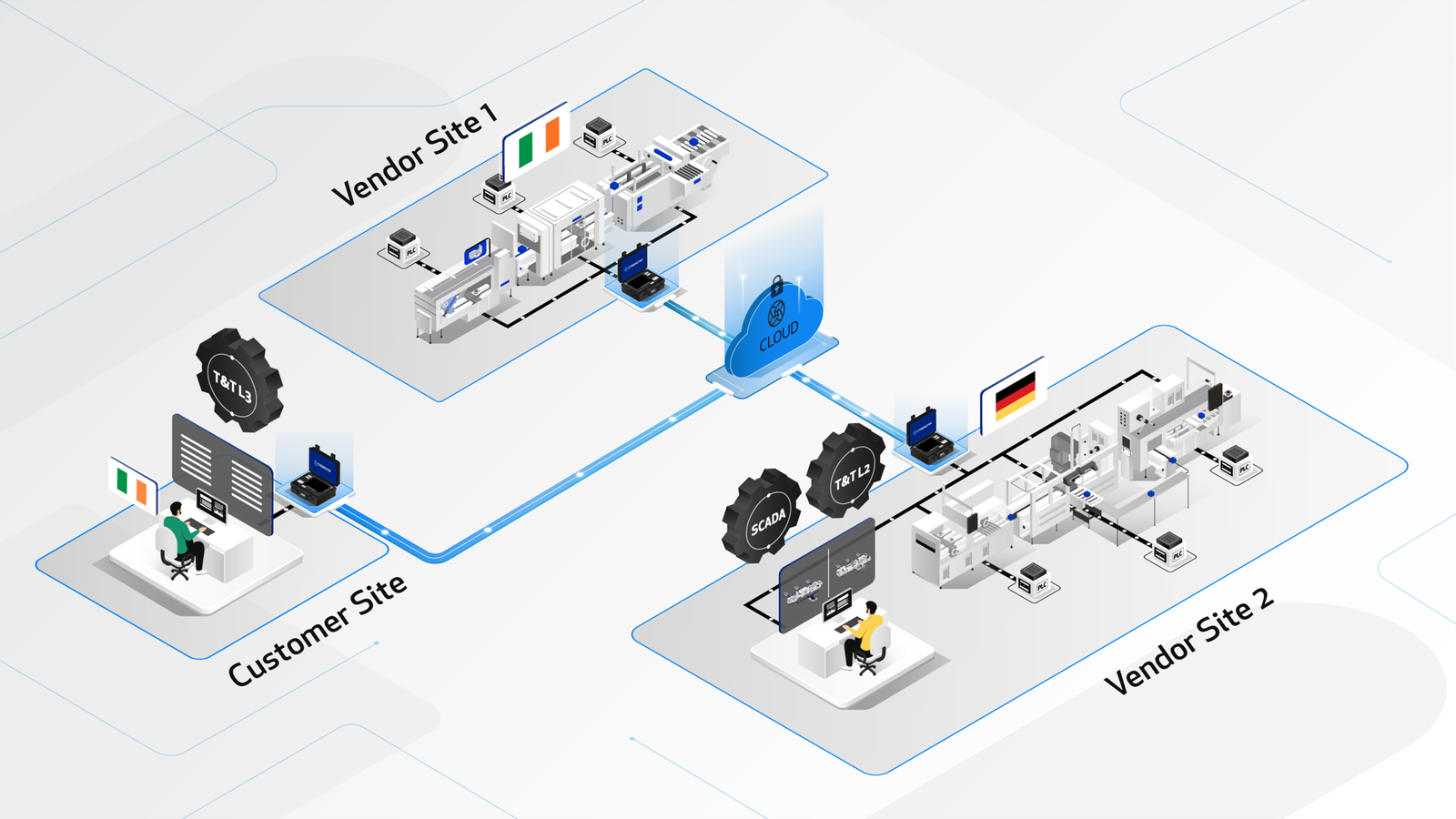
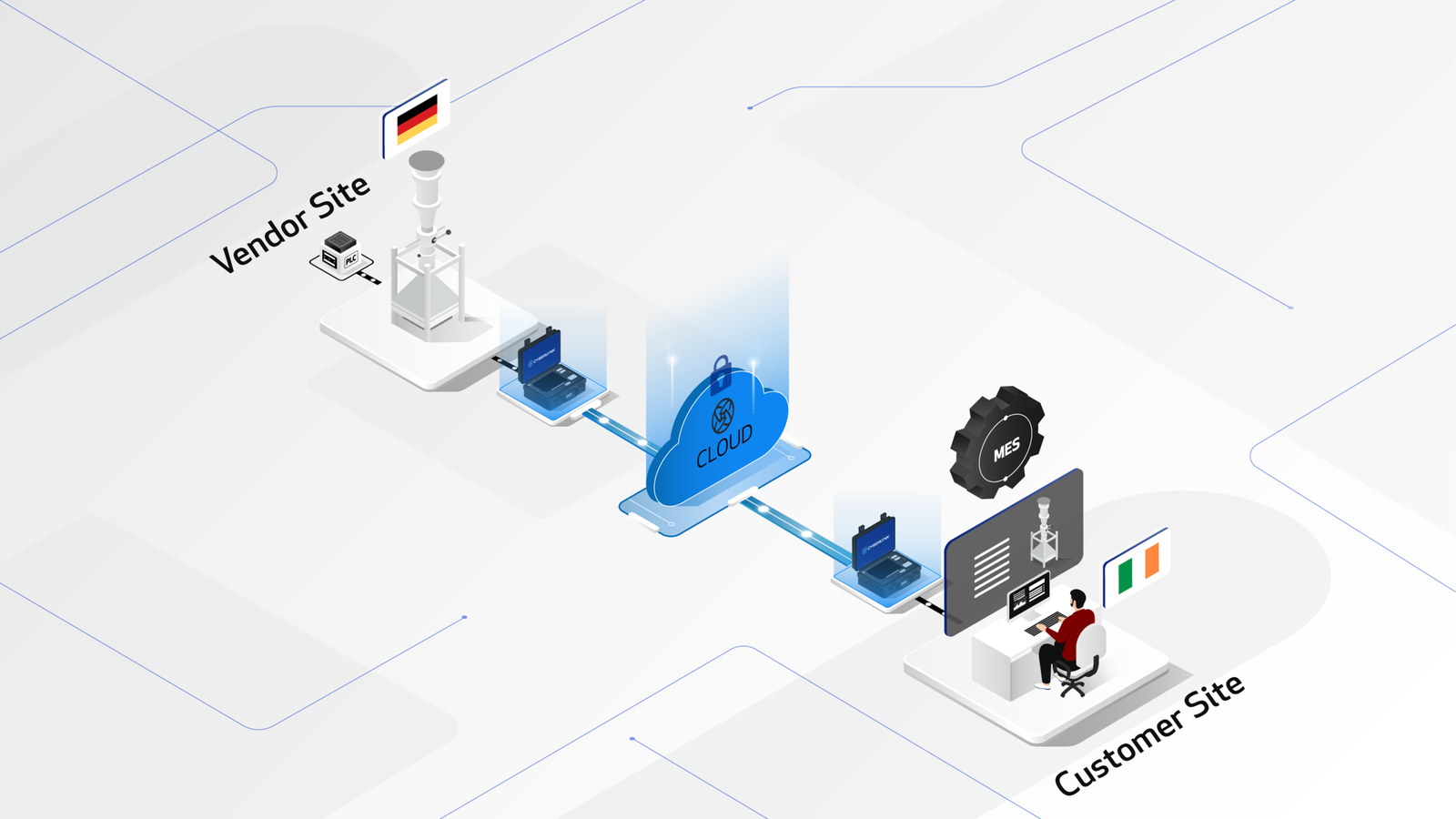
The CYBERLYNK device enables secure, plug-and-play connectivity between machines and software systems across different locations, allowing SAT-level network integration testing to be performed during the FAT stage.
By enabling pre-SAT integration at the vendor site, CYBERLYNK allows real production workflows, data exchanges, and control interactions to be tested much earlier in the project — when issues are faster, cheaper, and safer to resolve.
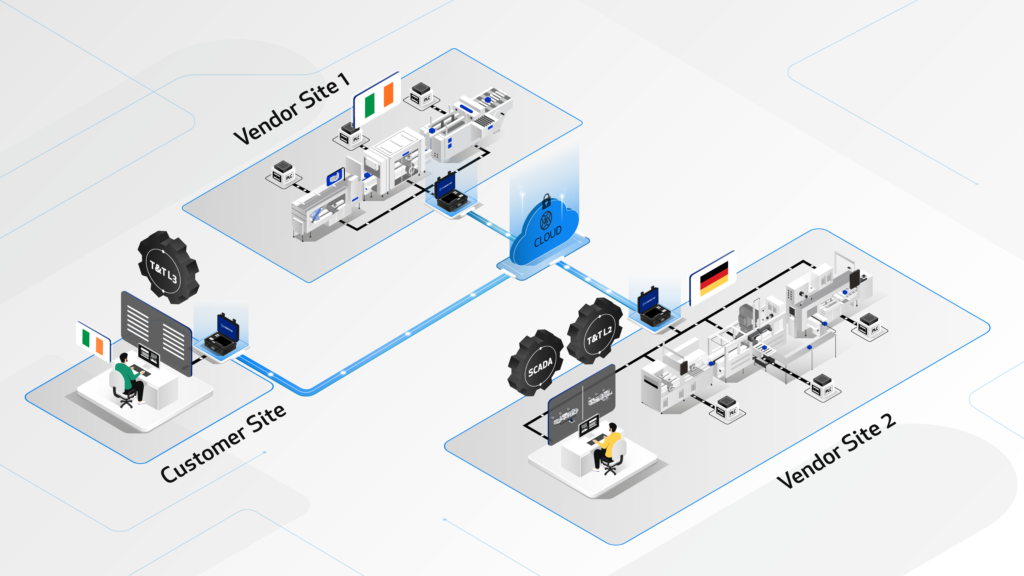
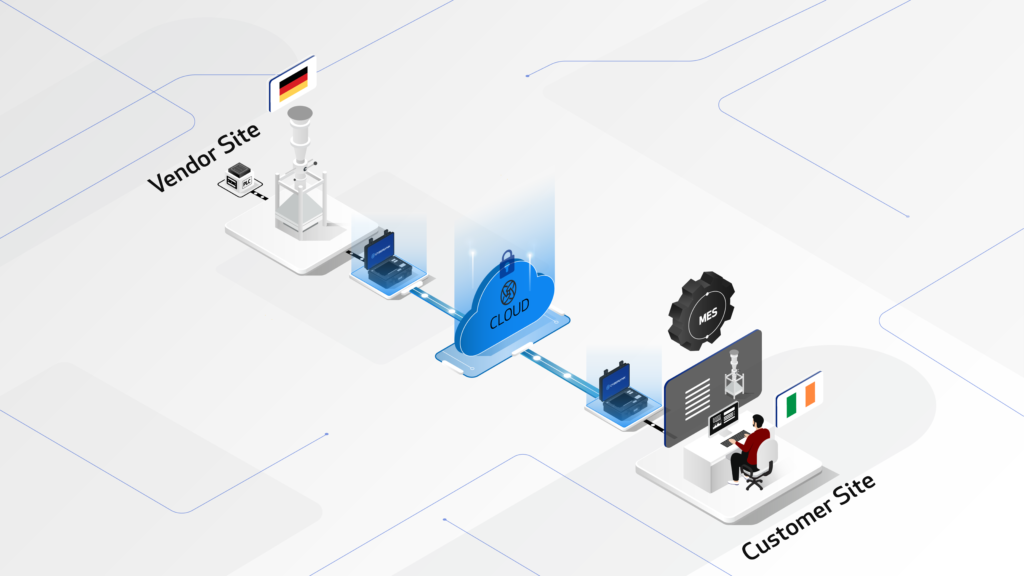
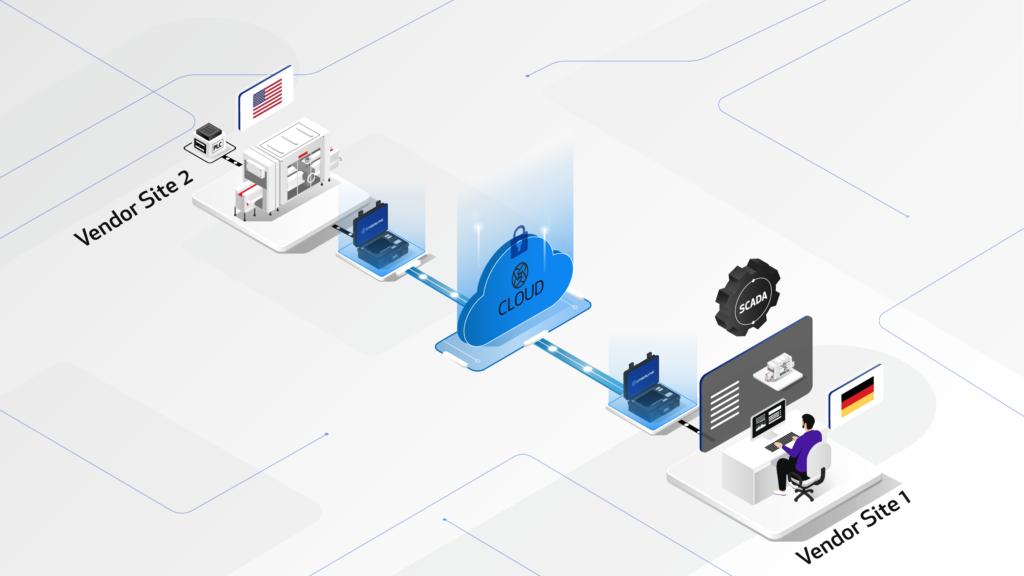
CYBERLYNK has been successfully deployed across complex pharmaceutical manufacturing projects in Europe and the USA involving MES, SCADA, Track & Trace, and multi-vendor production lines. By shifting integration testing earlier in the project lifecycle, customers achieve faster production readiness, reduced risk during SAT, and greater confidence that systems will perform as expected from day one.
The result is not just faster commissioning — but a more predictable, transparent, and resilient approach to delivering modern manufacturing systems.
To get started, send us a message via our contact form or give us a call expressing your interest in using CYBERLYNK for your project. Once we receive some initial details — such as the number of machines or systems involved and their locations — we’ll arrange a free consultation to discuss your requirements. Based on this, we’ll provide you with a tailored Service Level Agreement (SLA) and quotation.
CYBERLYNK can be implemented at any stage of a project, either as part of a planned approach or introduced into an ongoing project.
While CYBERLYNK delivers value and de-risks integration in all cases, the time-related benefits — including accelerated time to production — are maximised when CYBERLYNK is incorporated during the planning stage of the project.
CYBERLYNK enables earlier integration testing, and issue detection by connecting systems before physical installation. This helps reduce commissioning risk, shorten time-to-production, improve transparency across stakeholders, and avoid costly late-stage changes during SAT, or site commissioning.