

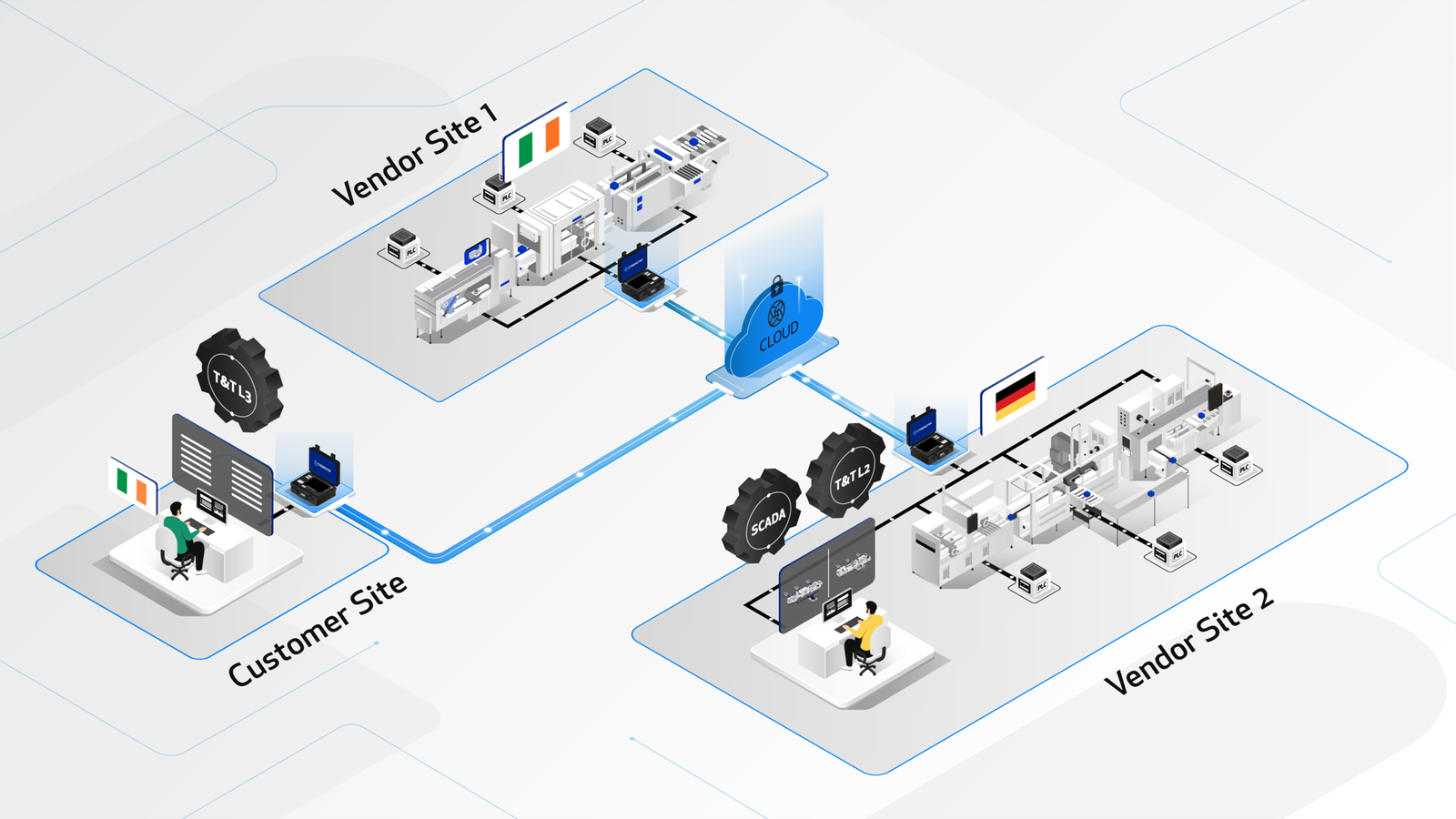
CYBERLYNK enabled full remote Track & Trace and SCADA integration across multiple vendor FAT locations in Ireland and Germany. By testing end-to-end line behaviour earlier in the project, critical integration issues were identified and resolved before delivery, accelerating production readiness by 7.5 weeks.
Complex pharmaceutical production lines are often assembled across multiple vendor locations, with Track & Trace and SCADA integration traditionally deferred until Site Acceptance Testing (SAT). This fragmented approach limits visibility of end-to-end system behaviour and increases the risk of late-stage integration failures.
Pharma Manufacturer
Factory Acceptance Testing (FAT)
Ireland (2 locations) ↔ Germany
Multi-site Pre-SAT integration
CYBERLYNK securely connected the customer’s Track & Trace (L3) system with the Start of Line at a vendor FAT site in Ireland and the End of Line at a separate vendor FAT site in Germany. This enabled full remote integration testing of SCADA and Track & Trace workflows across geographically distributed equipment before system delivery.
*During remote integration testing, a Track & Trace serialisation issue was identified with pallet-by-pallet data upload during batch execution. This issue was resolved by the vendor, followed by re-execution of the Track & Trace FAT, leading to full acceptance by the customer before delivery.
Time Saved:
7.5 weeks


We denounce with righteous indignation and dislike men who are so beguiled and demor alized by the charms of pleas ure of the moment, so blinded by desire. Neque qui is dolor emr ipsum quia dolor eque porro quisquam est.Tortor montes platea iacu lis posuere per mauris.